
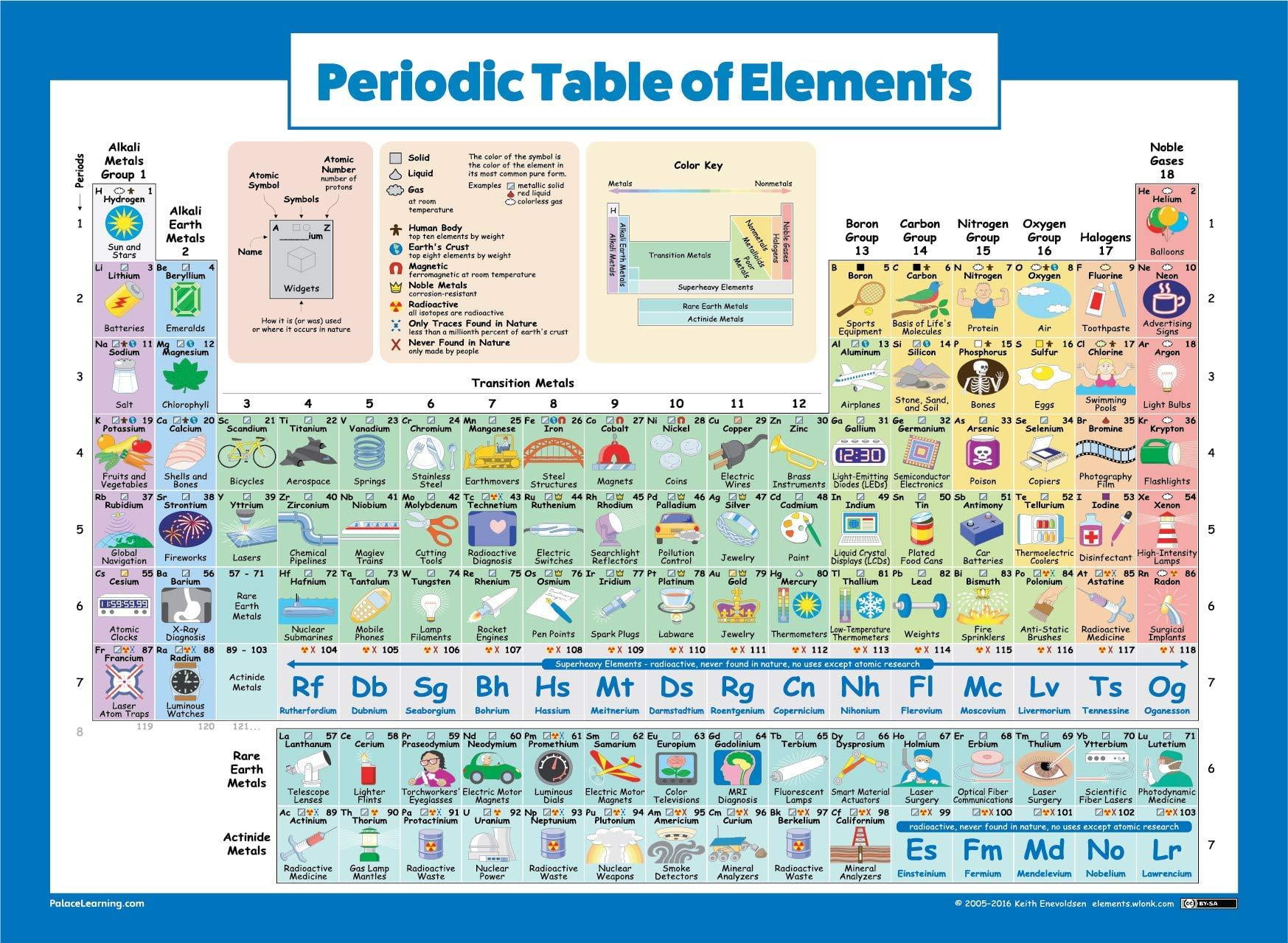
This is at odds with some other heavy elements, in which the neutron rings are well-defined.įor Oganessian, these theoretical predictions about the element have come as a surprise. Unlike oganesson’s protons, which are predicted to be in distinct shells in the nucleus, the element’s neutrons are expected to mingle. Experimental evidence for a “bubble nucleus” has been found for an unstable form of silicon ( SN: 11/26/16, p. But the sheer number of oganesson’s protons - 118 - may help the particles overcome this force, creating a bubble with few protons at the nucleus’s center, researchers say. Protons inside an atom’s nucleus repel one another due to their like charges, but typically remain bound together by the strong nuclear force. The periodic table of the elements was first introduced in the mid-19th century by Dmitri Mendeleev, who originally represented the elements as periodic. At room temperature, scientists expect that these oganesson atoms could clump together in a solid, unlike any other noble gases. Oganesson’s electron configuration could also let atoms of the element stick together, instead of just bouncing off one another as gas atoms typically do. As a result, the element could be chemically reactive.
/PeriodicTableWallpaper-56a12a3a3df78cf772680422.jpg)
But because of how its electrons are configured, oganesson is the only noble gas that’s happy to both give away its electrons and receive electrons. On the periodic table, oganesson is grouped with the noble gases, which tend not to react with other elements.


 0 kommentar(er)
0 kommentar(er)
